Amphiboles
Amphiboles, from the Greek amphibolos, meaning ambiguous, was named by the famous French crystallographer and mineralogist René-Just Haüy in allusion to the great variety of composition and appearance shown by this mineral group. There are 5 major groups of amphiboles leading to 76 chemically defined end-member amphibole compositions according to the British mineralogist Bernard E. Leake. Because of the wide range of chemical substitutions permissible in the crystal structure, amphiboles can crystallize in igneous and metamorphic rocks with a wide range of bulk chemistries. Typically, amphiboles form as long prismatic crystals, radiating sprays, and asbestiform (fibrous) aggregates; however, without the aid of chemical analysis, it is difficult to megascopically identify all but a few of the more distinctive end-member amphiboles. The combination of prismatic form and two diamond-shaped directions of cleavage at about 56° and 124° is the diagnostic feature of most members of the amphibole group.The complex chemical composition of members of the amphibole group can be expressed by the general formula:
A0-1B2C5T8O22(OH,F,Cl)2
where A = Na, K; B = Na, Zn, Li, Ca, Mn, Fe2+, Mg; C = Mg, Fe2+, Mn, Al, Fe3+, Ti, Zn, Cr; and T = Si, Al, Ti. Nearly complete substitution may take place between sodium and calcium and among magnesium, ferrous iron, and manganese (Mn). There is limited substitution between ferric iron and aluminum and between titanium and other C-type cations. Aluminum can partially substitute for silicon in the tetrahedral (T) site. Partial substitution of fluorine (F), chlorine, and oxygen for hydroxyl (OH) in the hydroxyl site is also common. The complexity of the amphibole formula has given rise to numerous mineral names within the amphibole group. In 1997 Leake presented a precise nomenclature of 76 names that encompass the chemical variation within this group. The mineral nomenclature of the amphiboles is divided into four principal subdivisions based on B-group cation occupancy: (1) the iron-magnesium-manganese amphibole group, (2) the calcic amphibole group, (3) the sodic-calcic amphibole group, and (4) the sodic amphibole group.
Numerous common amphiboles can be represented within the Mg7Si8O22(OH)2 (anthophyllite), Fe7Si8O22(OH)2 (grunerite) and Ca7Si8O22(OH)2 (hypothetical pure calcium amphibole) compositional field (Fig.1). This diagram is commonly referred to as the amphibole quadrilateral.
Complete substitution extends from tremolite [Ca2Mg5Si8O22(OH)2] to ferro-actinolite [Ca2Fe5Si8O22(OH)2]. Actinolite is the intermediate member of the tremolite-ferro-actinolite series. The compositional range from Mg7Si8O22(OH)2 to about Fe2Mg5Si8O22(OH)2is represented by the orthorhombic amphibole known as anthophyllite. The monoclinic cummingtonite-grunerite series exists from about Fe2Mg2Si8O22(OH)2 to Fe7Si8O22(OH)2. Intermediate amphibole compositions do not exist between anthophyllite and the tremolite-actinolite series.
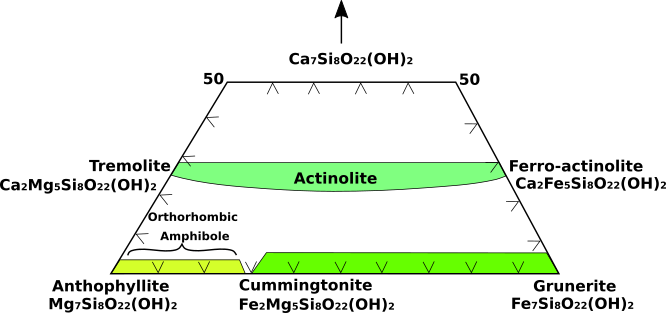
Fig.1: The amphibole quadrilateral composition diagram.
Compositional gaps also exist between the cummingtonite-grunerite series and other calcic amphiboles. Consequently, coexisting pairs of anthophyllite-tremolite and grunerite-ferroactinolite are found together in some rocks. Sodium-bearing amphiboles are represented by the glaucophane [Na2Mg3Al2Si8O22(OH)2] riebeckite [Na2Fe2+3Fe3+2Si8O22(OH)2] series. Additional sodium is contained in the A site of the structure of arfvedsonite [NaNa2Fe2+4Fe3+Si8O22(OH)2]. For amphiboles that are not precisely characterized by their chemistry, it is not possible to assign a specific name. Hornblende is the general name used for calcic amphiboles identified only by physical or optical properties.
The most common amphibole, hornblende, has very variable composition owing to significant substitution of Na+ and K+ in A site and Fe3+ and Al3+ in M 1-2-3 sites. Hornblende is the result of two main substitutions starting from tremolite: Si4+ + (Mg2+,Fe2+) ↔ 2Al3+, and Si4+ + ↔ A-site ↔ Al3+ + (Na+,K+). Hornblende includes following series: magnesiohornblende-ferrohornblende, tschermakite-ferrotschermakite, edenite-ferroedenite, pargasite-ferropargasite, and magnesiohastingsite-hastingsite. One way of classifying hornblende is to consider the A-site occupancy and the amount of Si in the T-site (Fig.2). In the diagram below yellow shows the range of observed composition with orange indicating the more abundant ones (Hb = hornblende (sensu stricto), Ts = tschermakite, Ed = edenite, and Pa = pargasite).
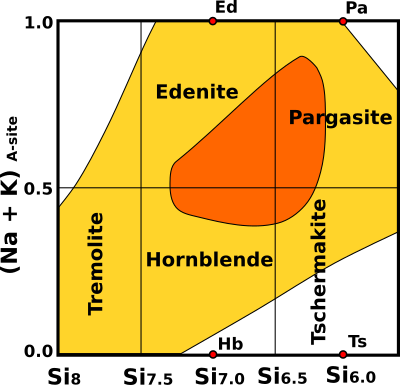
Fig.2: Hornblende classification diagram. Yellow shows the range of observed composition and orange indicate the more abundant ones (Hb = hornblende (sensu stricto), Ts = tschermakite, Ed = edenite, and Pa = pargasite).
Alkali amphiboles are divided into two groups based upon M4 and A site occupancies (Fig.3). If the M4 site contains <0.5 (Na+K) and there is no Na in the A site, the mineral is considered a low-Na alkali amphibole. If the M4 site contains >0.5 (Na+K) and there is Na in the A site, it is considered a high-Na alkali amphibole. There is no solid solution between calcic and alkali amphiboles. The two types of alkali amphiboles are further subdivided. Classification of low-Na alkali amphiboles is accomplished using the diagram below; the most common types are glaucophane and riebeckite. Alkali amphibole occurs primarily in sodic-metamorphic rocks and in alka-line igneous rocks.
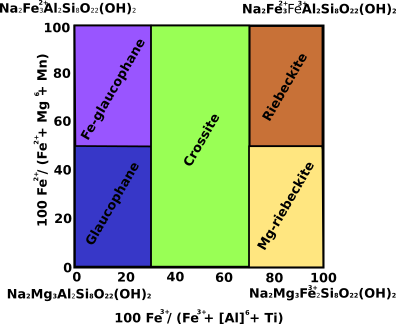
Fig.3: Alkali amphiboles classification diagram.
The amphiboles differ chemically from the pyroxenes in two major respects. Amphiboles have hydroxyl groups in their structure and are considered to be hydrous silicates that are stable only in hydrous environments where water can be incorporated into the structure as (OH)-. The second major compositional difference is the presence of the A site in amphiboles that contains the large alkali elements, typically sodium cations and at times potassium cations. The pyroxenes do not have an equivalent site that can accommodate potassium. The presence of hydroxyl groups in the structure of amphiboles decreases their thermal stability relative to the more refractory (heat-resistant) pyroxenes. Amphiboles decompose to anhydrous minerals (mainly pyroxenes) at elevated temperatures.
Structure
The amphibole structure consists of doubled (Si4O11)6- chains running parallel to c-axis (Fig.4). These chains are bonded to octahedral strips consisting of three regular octahedral sites (M1, M2, M3) and one larger 6- to 8-fold site (M4). In addition, there is an even larger 10- to 12-fold A site that is usually empty. OH- groups occur in the interiors of the rings in the double chains. The result is an I-beam structure like that of pyroxene (Fig.5). I-beam structure in amphiboles are approximately twice as wide as the equivalent t-o-t (tetrahedral-octahedral-tetrahedral) strips in pyroxenes, because of the doubling of the chains in the amphiboles, yielding typical near 60°-120° cleavage.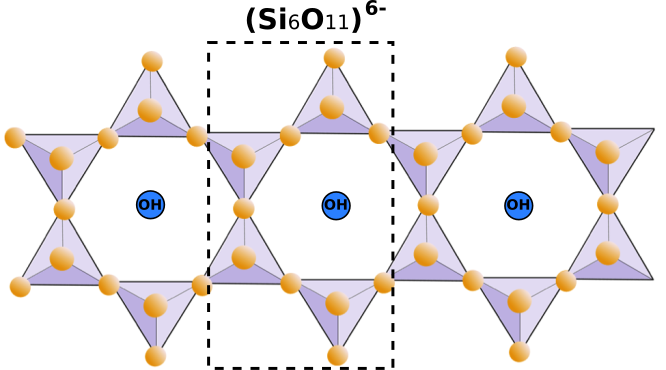
Fig.4: Amphibole double-chain structure (Si4O11)6-
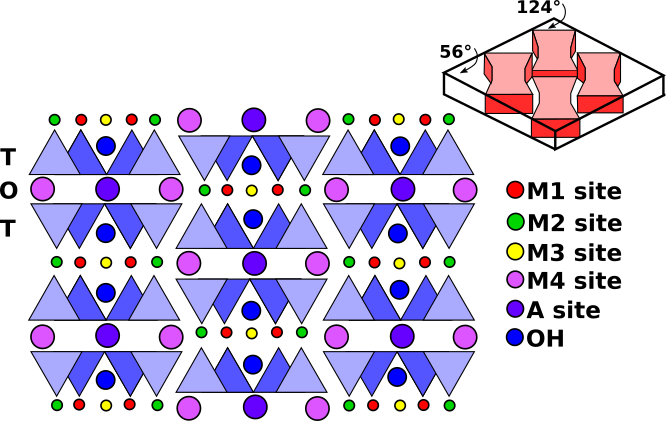
Fig.5: Schematic projection of the monoclinic amphibole structure on a plane perpendicular to the c axis showing the M1, M2, M3, M4 and A sites and I-beam structure yielding the typical near 60°-120° cleavage.
All the amphiboles, except anthophyllite are, monoclinic, and all show the excellent prismatic cleavage on (110). The angles between the cleavages, however are 56° and 124° making all amphiboles easy to distinguish from the pyroxenes. Looking at faces that show only a single cleavage trace would show inclined extinction, except in Anthophyllite.
Bibliography
• Cox et al. (1979): The Interpretation of Igneous Rocks, George Allen and Unwin, London.
• Howie, R. A., Zussman, J., & Deer, W. (1992). An introduction to the rock-forming minerals (p. 696). Longman.
• Le Maitre, R. W., Streckeisen, A., Zanettin, B., Le Bas, M. J., Bonin, B., Bateman, P., & Lameyre, J. (2002). Igneous rocks. A classification and glossary of terms, 2. Cambridge University Press.
• Middlemost, E. A. (1986). Magmas and magmatic rocks: an introduction to igneous petrology.
• Shelley, D. (1993). Igneous and metamorphic rocks under the microscope: classification, textures, microstructures and mineral preferred-orientations.
• Vernon, R. H. & Clarke, G. L. (2008): Principles of Metamorphic Petrology. Cambridge University Press.


.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
(21).jpg)
(22).jpg)
(31).jpg)
(9).jpg)
(11).jpg)
(10).jpg)
(12).jpg)
(13).jpg)
(19).jpg)