Perthite
Named for the nearby city of Perth in Ontario. The type locality is 9.5 km southwest of Perth. A perthite is an intimate intergrowth of sodic and potassic feldspar resulting from subsolidus exsolution (unmixing of two minerals). Strictly speaking a perthite has blebs or irregular lamellae of sodic feldspar within potassic alkali feldspar, however, the term perthite is often used to describe all types of exsolution in the feldspars. An antiperthite is an intergrowth arising due to exsolution where potassic feldspar is present as blebs or lamellae within a sodic feldspar. The term mesoperthite is used when sodic and potassic feldspars are in broadly equal anbundance. Perthite that can only be observed with the aid of a microscope is known as microperthite.Many minerals that show complete solid solution at higher temperatures do not show such solid solution at lower temperatures. When this is the case, the phenomenon of exsolution occurs. During the slow cooling experienced by a plutonic rock, alkali feldspar crystals have sufficient time to invert to structures that are stabler at low temperatures. Accordingly, plutonic rocks exhibit intra - crystalline textures in alkali feldspar crystals that are not seen in volcanic rocks.
Orthoclase (KAlSi3O8) - Albite (NaAlSi3O8) system
Dry, or with only small concentrations of water at relatively low P, this system is complicated by a large stability field of leucite (KAlSi2O6), Fig.1 a, whose composition cannot be expressed in terms of the components Ab and Or. However, at between 2 and 3 kbars under water-saturated conditions the stability field of leucite disappears and the system becomes truly binary. Solid solution is complete between the NaAlSi3O8 and KAlSi3O8 components; the solidus and liquidus form loops on each side of a minimum-melting composition. This minimum resembles a eutectic, in that evolved residual liquids move to it and there precipitate an alkali feldspar solid solution.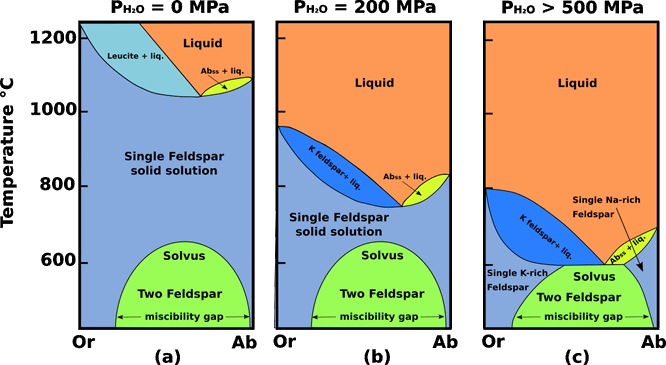
Fig.1 Orthoclase (KAlSi3O8) - Albite (NaAlSi3O8) system. (a) Dry conditions; in these conditions there is the presence of a large stability field of leucite. (b) At low (PH2O = 200 MPa) water-vapour pressure, complete solid solution exists at T > 700 ° C between KAlSi3O8 (Or) and NaAlSi3O8 (Ab). Crystallization of melt under these conditions generates a single homogeneous feldspar phase of intermediate composition, which exsolves to form perthite or antiperthite as it cools through the solvus into the 2-feldspar field, characteristic of hypersolvus granites. (c) High (PH2O > 500) water-vapour pressure, depresses the alkali feldspar solidus to the point where it intersects the solvus. Under these conditions no 1-feldspar field exists and therefore no homogeneous intermediate alkali feldspars can form; two separate feldspar species (one K-rich and one Na-rich) crystallize directly from the melt. Granites containing two distinct populations of potassic and sodic feldspars are referred to as subsolvus granites. Modified from Tuttle and Bowen (1958).
At PH2O = 200 MPa any feldspar precipitated from a melt is a homogeneous alkali feldspar solid solution. As any feldspar cools below the solidus, its isopleth eventually intersects the convex-upward solvus, below which the single feldspar unmixes, or exsolves, under equilibrium conditions, into two stable alkali feldspar solid solutions. These two feldspars produced by the exsolution process usually segregate within the original crystal as thin subparallel lamellae, forming the intergrowth known as perthite. At higher pressures, such as PH2O >500 MPa, in water-saturated systems the solidus loop is depressed so far that it intersects the solvus, forming an isothermal boundary line between the two-feldspar fields. In this case, the minimum in the liquidus is a eutectic.
The cause of exsolution is the solvus that divides the KAlSi3O8 - NaAlSi3O8 solid solution series. Its shape makes the degree of solid solution between orthoclase and albite highly temperature - sensitive: mutual solubility increases with temperature until, at temperatures above 700 ° C, solid solution spans the entire compositional range. This means that (at low water - vapour pressures) melts of granitic composition can crystallize a homogeneous alkali feldspar in the sanidine - anorthoclase range. The homogeneous hypersolvus feldspar persists down to 700 ° C or beyond, but, during slow cooling, as it crosses the solvus it will begin to unmix internally into microscopic lenses or patches of Na-rich feldspar dispersed in a K-feldspar host, or vice versa. The result will be a granite having a single population of perthite or microperthite crystals described as a hypersolvus granite. A typical example would be a granite or syenite emplaced at a very shallow level in the crust, having lost most of its volatile content by decompression during ascent.
The outcome is different when the feldspar crystallizes from melt under high PH2O, for example when a volatile rich magma crystallizes at depth. The solvus changes little in response to PH2O but the liquidus and solidus drop markedly at high PH2O. The solidus loop is depressed so far that it intersects the solvus, forming an isothermal boundary line between the two-feldspar fields. In this case, the minimum in the liquidus is a eutectic. The solid solution gap means that feldspars forming under these conditions crystallize as separate Or-rich and Ab- rich crystals. Granites containing two distinct populations of potassic and sodic feldspars are referred to as subsolvus granites.

Perthite unmixing in microcline. Evje, Norway. Image from sand atlas.

Perthite unmixing in microcline. Evje, Norway. Image from sand atlas.
Bibliography
• Cox et al. (1979): The Interpretation of Igneous Rocks, George Allen and Unwin, London.
• Howie, R. A., Zussman, J., & Deer, W. (1992). An introduction to the rock-forming minerals (p. 696). Longman.
• Le Maitre, R. W., Streckeisen, A., Zanettin, B., Le Bas, M. J., Bonin, B., Bateman, P., & Lameyre, J. (2002). Igneous rocks. A classification and glossary of terms, 2. Cambridge University Press.
• Middlemost, E. A. (1986). Magmas and magmatic rocks: an introduction to igneous petrology.
• Shelley, D. (1993). Igneous and metamorphic rocks under the microscope: classification, textures, microstructures and mineral preferred-orientations.
• Vernon, R. H. & Clarke, G. L. (2008): Principles of Metamorphic Petrology. Cambridge University Press.


.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)

.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)