Feldspathoid
The feldspathoid are family of rock-forming minerals consisting of aluminosilicates of sodium, potassium, or calcium and having too little silica to form feldspar. There is considerable structural variation, so it is not a true group. Feldspathoids take the places of feldspars in igneous rocks that are undersaturated with respect to silica or that contain more alkalis and aluminum than can be accommodated in the feldspars. Feldspathoids commonly occur with feldspars, and also with amphiboles, olivine, and pyroxenes, but never with quartz or other polymorphs of silica.Like the feldspars, they have framework structures that consist of silica and alumina tetrahedrons. Unlike the feldspars, however, the arrangements of the tetrahedrons differ from species to species, and the interstices may contain water and/or other simple or complex anions such as chlorine Cl-, carbonate (CO3)2-, or sulfate (SO4)2-, as well as sodium, potassium, and calcium. Consequently, different feldspathoids have somewhat different structures: some are isometric, others are hexagonal, and still others are tetragonal. Minerals of the feldspathoid group include nepheline, leucite, sodalite, and cancrinite.
They are considered to be the specific minerals of igneous alkalic rocks. The feldspathoids, along with minerals of the melilite group, are referred to as foids in the IUGS classification of igneous rocks. They do not occur in igneous rocks containing original free silica. They are, in fact, incompatible with quartz, as is quite apparent from the following equations:
(Na,K)AlSiO4 (nepheline) + 2SiO2 (quartz) = NaAlSi3O8 (albite)
and
and KAlSi2O6 (leucite) + SiO2 (quartz) = K(AlSi3O8) (K-feldspar)
Feldspathoids compositions can be displayed using a tetrahedron with quartz at the apex (Fig.1). The ranges of observed substitutions are shown by the colored fields.
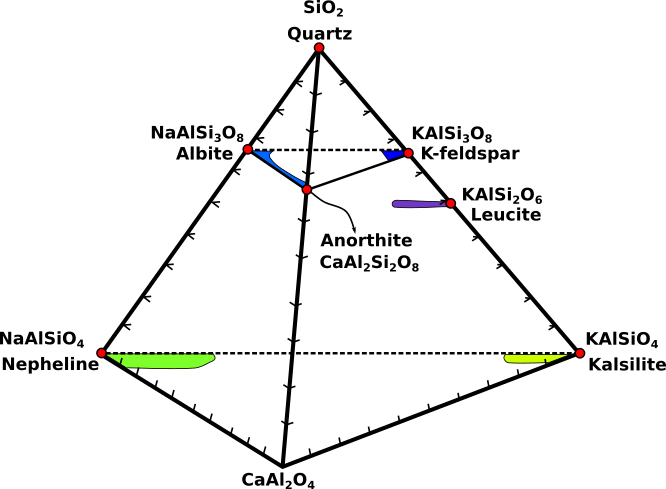
Fig.1: Feldspathoids minerals compared to feldspar minerals.
Nepheline (Na,K)AlSiO4, also called nephelite, or eleolite, is the most common feldspathoid mineral. Nepheline is the characteristic mineral of alkaline plutonic rocks, particularly nepheline syenites. It occurs in beautiful crystal form with mica, garnet, and sanidine feldspar on Monte Somma, Vesuvius, Italy.
Leucite KAlSi2O6 occurs only in igneous rocks, particularly potassium-rich, silica-poor, recent lavas. Some important localities include Rome; Uganda; and Leucite Hills, Wyo., U.S. Leucite is used as a fertilizer in Italy (because of its high potassium content).
Sodalite Na4(AlSiO4)3Cl occurs with leucite and nepheline in such igneous rocks as nepheline syenite, trachyte, and phonolite. The sodalite group also contains hydroxy-sodalite (hydroxide substitutes for chloride), nosean Na8(AlSiO4)6 ∗ H2O, and haüyne (Na,Ca)4-8(AlSiO4)6(SO4,Cl)1-2. Nosean is a common constituent of volcanic ejecta. Haüyne varies in colour from white or gray to green or blue. The blue variety, lazurite, is the primary constituent of lapis lazuli; its depth of colour seems to increase with additional sulfide substitution.
Cancrinite Na6Ca2(AlSiO4)6(CO3) ∗ H2O is a rare feldspathoid mineral and occurs as an alteration product of nepheline and feldspar in nepheline-syenite and related rocks. Famous localities are Alnö, Sweden; Fen district, Norway; Kola, Russia; and Iron Hill, Colo., U.S.
Bibliography
• Cox et al. (1979): The Interpretation of Igneous Rocks, George Allen and Unwin, London.
• Howie, R. A., Zussman, J., & Deer, W. (1992). An introduction to the rock-forming minerals (p. 696). Longman.
• Le Maitre, R. W., Streckeisen, A., Zanettin, B., Le Bas, M. J., Bonin, B., Bateman, P., & Lameyre, J. (2002). Igneous rocks. A classification and glossary of terms, 2. Cambridge University Press.
• Middlemost, E. A. (1986). Magmas and magmatic rocks: an introduction to igneous petrology.
• Shelley, D. (1993). Igneous and metamorphic rocks under the microscope: classification, textures, microstructures and mineral preferred-orientations.
• Vernon, R. H. & Clarke, G. L. (2008): Principles of Metamorphic Petrology. Cambridge University Press.

