Olivine - (Fe,Mg)2SiO4
Olivines are an important rock-forming mineral group. Magnesium-rich olivines are abundant in low-silica mafic and ultramafic igneous rocks and are believed to be the most abundant constituent of the Earth’s upper mantle. Olivine also occurs in high-temperature metamorphic rocks, lunar basalts, and some meteorites. The name olivine derives from the unusual yellow green to deep bottle-green color of the magnesium-iron olivine series. Typically, the name olivine is given to members of the forsterite-fayalite solid-solution series. In addition to these magnesium and ferrous iron end-members, the olivine group contains manganese (tephroite), calcium-manganese (glaucochroite), calcium-magnesium (monticellite), and calcium-iron (kirschsteinite) end-members.The composition of most olivines can be represented in the system Ca2SiO4 - Mg2SiO4 - Fe2SiO4 (Fig.1). The most abundant olivines occur in the system from forsterite (Mg2SiO4) to fayalite (Fe2SiO4). Most of the naturally occurring olivines are intermediate in composition to these two end-members and have the general formula (Mg, Fe)2SiO4. Members of the series monticellite (CaMgSiO4) to kirschsteinite (CaFeSiO4) are rare. Minor elements such as aluminum, nickel, chromium, and boron can substitute in olivine.
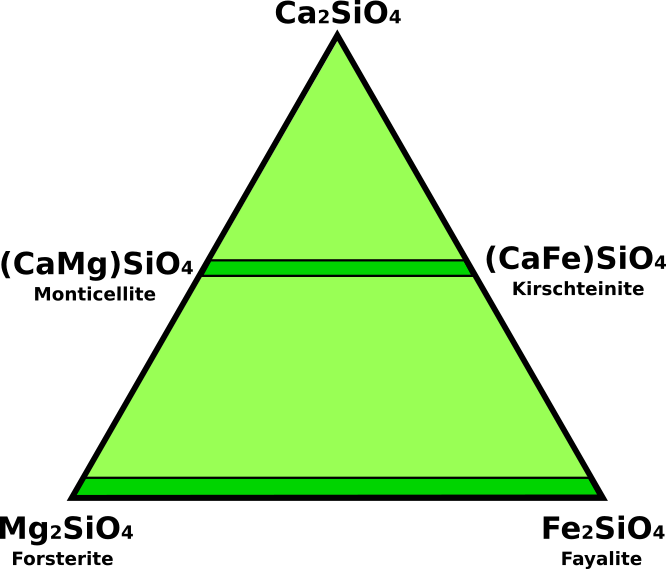
Fig.1: Ternary system Ca2SiO4 - Mg2SiO4 - Fe2SiO4 for olivine minerals.
The name forsterite is restricted to those species with no more than 10 percent iron substituting for magnesium; fayalite (from Fayal Island in the Azores) is restricted to species with no more than 10 percent magnesium substituting for iron. Compositions intermediate to these series end-members are identified by FoxFay, which is an expression of the molar percentage of each compound. For example, Fo70Fa30 denotes a composition of olivine that is 70 percent forsterite. The notation is shortened to Fo70. In addition to the forsterite-fayalite series, other complete solid-solution series exist among the various olivine minerals. The monticellite-kirschteinite series has larger unit cell than forsterite-fayalite series, accommodating the larger Ca cation.
Structure
All olivines crystallize in the orthorhombic crystal system. Olivine is classified as a nesosilicate which has isolated (SiO4)4- tetrahedrons bound to each other only by ionic bonds from interstitial cations. The olivine structure (Fig.2) consists of Isolated (SiO4)4- tetrahedra pointing alternately up and down along rows parallel to c axis. Mg2+ and Fe2+ occur in octahedral coordination (M-cations) in two distinct octahedral sites: a distorted M1 site and a more regular M2 site. Layers occur parallel to (100) with edge-sharing octahedra cross-linked by isolated (SiO4)4- tetrahedra. Mg2+ and Fe2+ appear to occupy the M1 or M2 sites without preference in the Mg-Fe series. In CaMgSiO4 olivine, Ca2+ prefers M2 site with Mg2+ in the M1 site.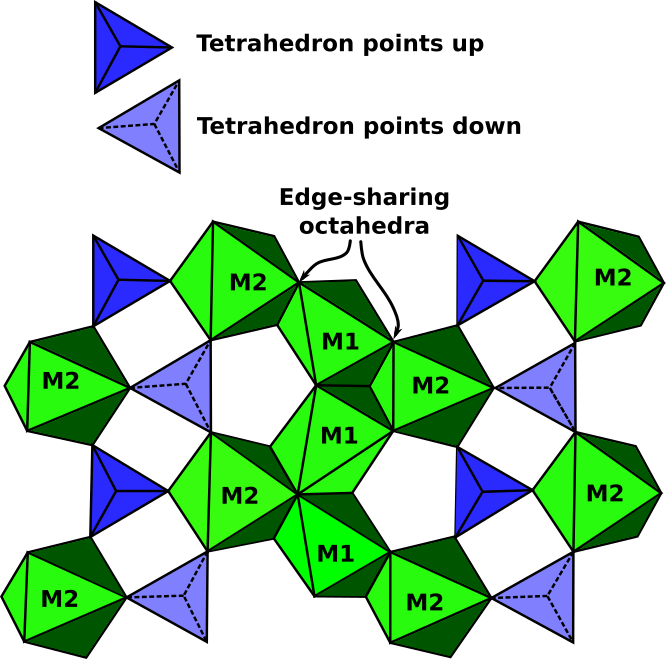
Fig.2: Idealized structure of olivine projected perpendicular to the a-axis showing the positions of the M1 and M2 octahedral sites
Olivines are found most commonly in mafic and ultramafic igneous rocks such as peridotites, dunites, gabbros, and basalts. Forsteritic olivines, which have 88 to 92 percent forsterite, are the dominant phase of dunites and are common in peridotites. Gabbros and basalts typically contain forsteritic to intermediate-composition olivine ranging from 50 to 80 percent forsterite. Olivine is typically associated with calcic plagioclase, magnesium-rich pyroxenes, and iron-titanium oxides such as magnetite and ilmenite. This mineralogical association is diagnostic of the relatively high temperatures of crystallization of mafic rock types.
Magnesium-rich olivine is unstable in a high-silica environment and is never found in equilibrium with quartz. The chemical reaction that precludes the stable coexistence of forsterite and quartz due to the formation of the orthopyroxene enstatite in the presence of excess silica is:
Mg2SiO4 (forsterite) + SiO2 (quartz) → Mg2Si2O6 (orthopyroxene)
Fayalite, however, can coexist in equilibrium with quartz in iron-rich granites and rhyolites. Olivines richer in iron than Fa50 are less common; they do occur in the iron-enriched layers of some intrusive rocks, however. Fayalite itself occurs in small amounts in some silicic volcanic rocks as a primary mineral in rhyolites and obsidians. It also occurs in acidic plutonic rocks such as granites in association with iron-enriched amphiboles and pyroxenes.
Olivine in the Earth mantle
At the high temperatures and pressures found at depth within the Earth the olivine structure is no longer stable. Below depths of about 410 km olivine transforms into wadsleyite and, at about 520 km depth, wadsleyite transforms into ringwoodite, which has the spinel structure. At a depth of about 660 km, ringwoodite decomposes into silicate perovskite (Mg,Fe)SiO3 and ferropericlase (Mg,Fe)O. These phase transitions lead to a discontinuous increase in the density of the Earth's mantle that can be observed by seismic methods (Fig.3).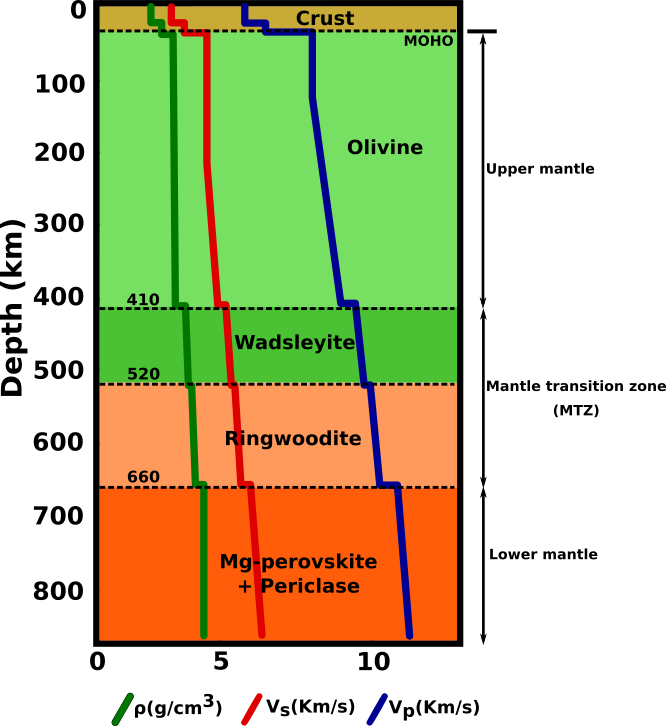
Fig.3: Seismic and density structures of the lower mantle showing the major discontinuities.
Alteration
Olivine is highly subject to weathering, hydrothermal alteration, and metasomatic alteration. Alteration product are often complex mixture of fine-grained minerals that are not distinguished by optical means. Some characteristic forms of olivine alteration are:Serpentine: Serpentine alteration is common in plutonic or metamorphic rocks, it being along fracture, as the result of hydrothermal or deuteric processes and may completely convert olivine rocks in a serpentine rock, with a structure characteristic of the olivine fracture pattern ("mesh" structure). Serpentine alteration can be described by the reaction:
2Mg2SiO4 (olivine) + 3H2O = Mg3Si2O5(OH)2 (serpentine) + Mg(OH)2 (brucite).
If CO2 is present in the system:2Mg3Si2O5(OH)4 (serpentine) + 3CO2 = Mg3Si4O10(OH)2 (talc) + 3MgCO3 (magnesite) + 3H2O.
Iddingsite and bowlingite: Iddingsite and bowlingite alteration are most characteristic of oxidation, deuteric alteration or weathering. Iddingsite appears as reddish-brown replacement of olivine, along fracture, rims, or complete replacement. Iddingsite may appears optically homogeneous, with high relief, and high interference colors. Bowlingite is a fine-grained aggregate containing smectite, goethite, chlorite, calcite, silica, talc.
Chlorophaeite: Chlorophaeite alteration is also characteristic of volcanic or shallow intrusion, this fine-grained aggregate tends to be isotropic. Probably contain limonite, chlorite and serpentine.
Optical Properties:
• Form: Six sides circular section on (001), rectangular section on (010), six or eight sides section elongated on c in (100) section.
• Color: Colorless or pale green-yellow with increasing Fe.
• Relief: High.
• Interference colors: Strong, III order colors.
• Cleavage: Rather poor on (010) and (110).
.png)
Green forsterite. San Carlos Apach Riservation, Arizona, U.S.A. From D. Nishio-Hamane.
.png)
Dunitic xenolith. San Carlos Apach Riservation, Arizona, U.S.A. From James St. John.

olivine xenolith, in basalt, 1800 lava flow, Hualalai Volcano, Hawaii County, Hawaii. From Alan Cressler.

Red fayalite. Ochtendung, Eifel, Germany. From Wikipedia.

Brown monticellite crystals in a carbonatite. Magnet Cove, USA. From RRUFF.

Red tephroite crystals. Franklin, New Jersey, USA. From RRUFF.
Bibliography
• Cox et al. (1979): The Interpretation of Igneous Rocks, George Allen and Unwin, London.
• Howie, R. A., Zussman, J., & Deer, W. (1992). An introduction to the rock-forming minerals (p. 696). Longman.
• Le Maitre, R. W., Streckeisen, A., Zanettin, B., Le Bas, M. J., Bonin, B., Bateman, P., & Lameyre, J. (2002). Igneous rocks. A classification and glossary of terms, 2. Cambridge University Press.
• Middlemost, E. A. (1986). Magmas and magmatic rocks: an introduction to igneous petrology.
• Shelley, D. (1993). Igneous and metamorphic rocks under the microscope: classification, textures, microstructures and mineral preferred-orientations.
• Vernon, R. H. & Clarke, G. L. (2008): Principles of Metamorphic Petrology. Cambridge University Press.


.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)

.jpg)
.jpg)
.jpg)
.jpg)

.jpg)

.jpg)
.jpg)
.jpg)
.jpg)
(48).jpg)
(49).jpg)
(50).jpg)
(53).jpg)
(55).jpg)
(57).jpg)