Diopside-Taramellite marble
The term marble in geology is restricted to metamorphic rocks in which the carbonate minerals have recrystallized. This process generally increases the average grain size, which gives marble its sparkling appearance. The word "marble" derives from the Greek (màrmaron) "crystalline rock", "shining stone”. The majority of marbles are dominated by calcite and/or dolomite (marbles dominated by dolomite are termed dolomarbles).Marbles are formed by regional, and less commonly thermal, metamorphism of relatively pure limestone and dolomite, and in general, metamorphism of relatively pure limestone and dolomite yields generally phaneritic marble and dolomite marble, or dolomarble, respectively. Impure carbonate protoliths that contain variable amounts of quartz sand grains and shaly material may have, in addition to calcite and dolomite, quartz, diopside, tremolite, talc, phlogopite, wollastonite, calcic plagioclase, vesuvianite, forsterite, and grossular-andradite. Relict bedding may be preserved. Gray coloration, often uneven and streaked, in otherwise pure marbles is produced by minute specks of graphite or other minerals. But graphite and carbonaceous material can react with water under metamorphic conditions, liberating CO2 and CH4 that leaves the rock snow-white.
Regional metamorphism of dolomite and calcite limestone
Impure dolomitic and calcitic limestone sediments may be metamorphosed by regional metamorphism; the regional metamorphism of such impure calcitic and dolomitic limestones produces a variety of silicate minerals.Dolomite limestones
The P-T-X relationships of dolomite-rich marbles in orogenic belts are shown in Fig.1.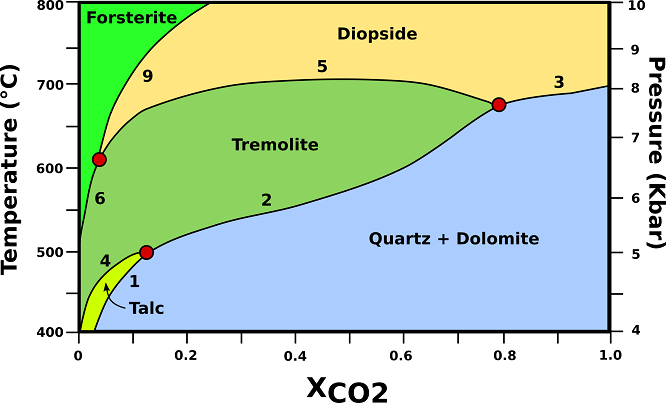
Fig.1: P-T-X phase relationships in dolomite marbles containing excess dolomite and calcite. The vertical axis represents an orogenic geotherm characteristic of regional metamorphism in collision belts. (1) 3Dol + 4Qtz + H20 → Tlc + 3Cal + 3C02; (2) 5Dol + 8Qtz + H20 → Tr + 3Cal + 7C02; (3) Dol + 2Qtz → Di + 2C02; (4) 2Tlc + 3Cal → Tr + Dol + C02 + H20; (5) 3Cal + Tr → Dol + 4Di + H20 + C02; (6) 11Dol +Tr → 13Cal + 8Fo + 9C02 + H20; (9) 3Dol + Di → 4 Cal + 2Fo + 2C02; Cal= calcite; Di= diopside; Dol= dolomite; Fo= forsterite; Qtz= quartz; Tlc= talc; Tr= tremolite. Modified from Bucher, K., & Grapes, R. (2011).
♦ Talc: Talc-bearing marbles are restricted to T <500 °C at P <5 kbar. However, talc in marbles of orogenic belts is usually associated with late hydrothermal activity (H20-rich fluids) or it is of retrograde origin. Talc is the only metamorphic mineral in dolomites at lower metamorphic grades. Tremolite is, therefore, usually the first recognizable metamorphic mineral in marbles. Talc is removed from the marbles by reaction (4).
♦ Tremolite: first occurs of tremolite is at about 500 °C and 5 kbar. The first occurrence of tremolite represents an excellent mappable isograd in the field and coincides approximately with the beginning of the amphibolite facies. Reaction (2) will continue to produce tremolite with increasing temperature. Finally, the rock will run out of the reactant quartz and will enter the tremolite field. The assemblage Dol + Cal + Tr is the characteristic assemblage in lower to middle amphibolite facies dolomite marbles. At a typical mid-amphibolite facies temperature (600 °C, 6.5 kbar).
♦ Diopside: diopside forms in dolomite marbles from reactions (3) and (5), and from the reaction at the invariant point Qtz + Tr + Di (+ Dol + Cal). The position of this invariant point defines the lowest temperature of possible occurrence of diopside in dolomite marbles; the first occurrence of diopside represents a sharp mappable boundary in the field and coincides with a temperature of about 670 °C. If a rock does not reach the invariant point along a prograde path because reaction (2) consumed all quartz at lower temperature, then diopside will form from reaction (5). This reaction separates tremolite from diopside in the field. Along a collision-type P-T path, no tremolite bearing dolomite marbles can be stable at T > 720 °C.
♦ Forsterite: Diopside will be removed from dolomitic marbles by reaction (9). The reaction replaces the Dol+Cal+Di assemblage of the diopside field by the Dol+Cal+ Fo assemblage of the forsterite field. At temperatures <800 °C, forsterite can only be produced by interaction of the marble with an externally derived H20-rich fluid phase (e. g., along fractures or shear zones, veins). Closed-system marbles will be free of forsterite if metamorphism occurred at P-T conditions of regional metamorphism.
Calcite limestones
The P-T-X relationships of calcite-rich marbles in orogenic belts are shown in Fig.2. Dolomite is the limiting mineral in reactions (1), (2), and (3) for these compositions.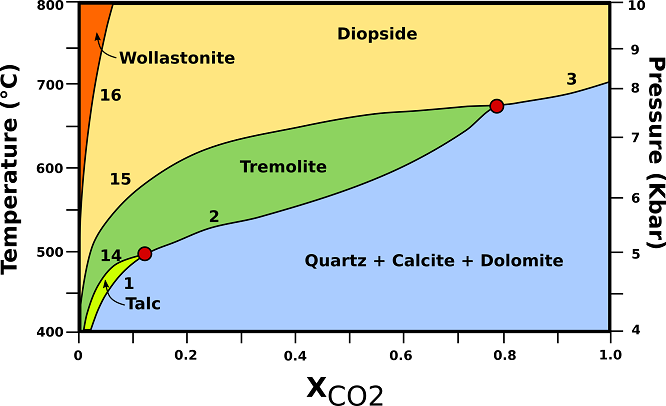
Fig.2: P-T-X phase relationships in calc-silicate marbles containing excess quartz and calcite. The vertical axis represents an orogenic geotherm characteristic of regional metamorphism in collision belts. (1) 3Dol + 4Qtz + H20 → Tlc + 3Cal + 3C02; (2) 5Dol + 8Qtz + H20 → Tr + 3Cal + 7C02; (3) Dol + 2Qtz → Di + 2C02; (14) 5Tlc + 6Cal + 4Qtz → 3Tr+ 6C02 + 2H20; (15) 3Cal + 2Qtz + Tr → 5Di + H20 + 3C02; (16) Cal + Qtz → Wo + C02; Cal= calcite; Di= diopside; Qtz= quartz; Tlc= talc; Tr= tremolite; Wo = wollastonite. Modified from Bucher, K., & Grapes, R. (2011).
♦ Tremolite: The tremolite-producing reaction (2) will consume all dolomite and the resulting assemblage, after completion of reaction (2), is Cal+Qtz+Tr. Here, tremolite will be removed from the rocks at higher grades by reaction (15). The tremolite field for these rocks is smaller in size than for dolomite-rich marbles.
♦ Diopside: diopside is usually produced by reaction (15), and it may appear in calcite marbles at lower temperature than in dolomite marbles (650°C). The highest-grade assemblage in calcsilicate marbles is Cal + Qtz + Di. In contrast to dolomite-rich rocks, there is very little overlap between the tremolite and the diopside field in calcite marbles.
♦ Wollastonite: wollastonite does not form in regional metamorphic rocks under closed system conditions. Even under granulite facies conditions the assemblage Cal + Qtz remains stable. Fig.2 shows that wollastonite may only form from reaction (16) by interaction of the marble with an H20-rich fluid. Such externally derived fluids may invade the marbles along fractures or shear zones.
Contact Metamorphism of Dolomite and Calcite limestone
Dolomitic and calcitic limestone sediments may also be metamorphosed by adding heat from an igneous heat source at shallow levels in the crust. Commonly, the intrusive body is of granitic, granodioritic or quartz-dioritic composition and the temperature at the contact to the country rocks is in the order of 600-700 °C.Dolomite limestones
Stability relationships of siliceous dolomites at 2 kbar are shown in Fig.3. The size (thickness) of the distinct mineralogical zones in the aureole depends on the heat given off by the pluton that, in turn, depends on the size of the intrusion, the composition of the magma and its temperature. Typically, contact effects of shallow-level intrusions can be recognized in the country rocks to distances ranging from a few hundred meters to 1 or 2 km.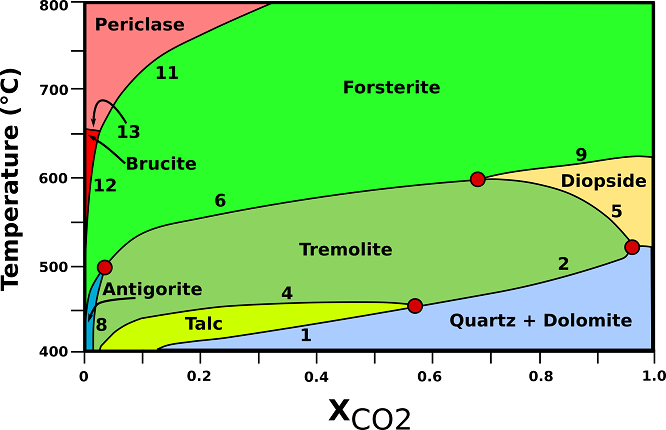
Fig.3: T -X phase relationships in dolomite marbles containing excess dolomite and calcite at a constant pressure of 2 kbar (contact metamorphic aureoles). (1) 3Dol + 4Qtz + H20 → Tlc + 3Cal + 3C02; (2) 5Dol + 8Qtz + H20 → Tr + 3Cal + 7C02; (3) Dol + 2Qtz → Di + 2C02; (4) 2Tlc + 3Cal → Tr + Dol + C02 + H20; (5) 3Cal + Tr → Dol + 4Di + H20 + C02; (6) 11Dol +Tr → 13Cal + 8Fo + 9C02 + H20; (8) 107Dol + 17Tr + 107H20 → 141Cal + 4Atg + 73C02; (9) 3Dol + Di → 4 Cal + 2Fo + 2C02; (10) 20Dol + Atg → 20Cal + 34Fo + 20C02 + 31H20; (11) Dol → Cal + Per + C02; (12) Dol + H20 → Cal + Brc + C02; (13) Brc → Per + H20; Atg= antigorite; Brc= brucite; Cal= calcite; Di= diopside; Dol= dolomite; Fo= forsterite; Per= periclase; Qtz= quartz; Tlc= talc; Tr= tremolite. Modified from Bucher, K., & Grapes, R. (2011).
♦ Tremolite: tremolite-bearing dolomites may develop from reactions (2), (4), or the reaction at the invariant point. The low-temperature limit for tremolite coincides with the upper limit for talc (450 °C). All tremolite will subsequently be removed from dolomite marbles by reactions (5) and (6). The maximum temperature for tremolite in dolomite marble is given by the position of the invariant point Tr + Di + Fo (+ Dol + Cal) at about 600°C. Reaction (6) produces forsterite in tremolite-bearing marbles in low-pressure aureoles.
♦ Forsterite: Forsterite appears in marbles in modally recognizable amounts at about 570-600°C, depending on the quartz content of the original dolomite. The assemblage Fo + Dol + Cal is characteristic of dolomites close to the contact to the intrusion. Reaction (6), if run to completion, results in the assemblage Dol + Cal + Fo in dolomite-rich rocks.
♦ Diopside: Diopside is restricted to a narrow T interval near 600°C under fluid-present conditions. It will form by reaction (5) or at the invariant point Qtz + Tr + Di (+ Dol + Cal).
♦ Periclase: Periclase forms usually from decomposition of dolomite according to reaction (11).
♦ Brucite: Brucite may form by direct decomposition of dolomites by reaction (12) or, more commonly, by retrograde hydration of previously formed periclase by the reversed reaction (13).
Calcite limestones
Calcite limestone undergo progressive transformations which can be described by the phase relationships shown in Fig.4.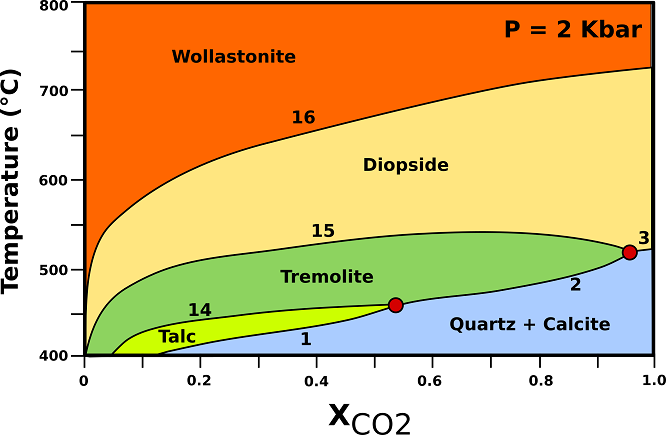
Fig.4: T-X phase relationships in calc-silicate marbles containing excess quartz and calcite at a constant pressure of 2 kbar (contact metamorphic aureoles). (1) 3Dol + 4Qtz + H20 → Tlc + 3Cal + 3C02; (2) 5Dol + 8Qtz + H20 → Tr + 3Cal + 7C02; (3) Dol + 2Qtz → Di + 2C02; (14) 5Tlc + 6Cal + 4Qtz → 3Tr+ 6C02 + 2H20; (15) 3Cal + 2Qtz + Tr → 5Di + H20 + 3C02; (16) Cal + Qtz → Wo + C02; Cal= calcite; Di= diopside; Qtz= quartz; Tlc= talc; Tr= tremolite; Wo = wollastonite. Modified from Bucher, K., & Grapes, R. (2011).
♦ Tremolite: Tremolite has a distinctly lower maximum temperature of occurrence compared with dolomite-rich rocks. Tremolite is replaced by diopside by reaction (15), which has its temperature maximum at 540°C.
♦ Diopside: diopside first appears in calc-silicate marbles further away from the intrusive contact than in dolomite marbles. The assemblage Cal + Di + Qtz is typical for calcite marbles near the contact to the intrusives.
♦ Wollastonite: Wollastonite may form in contact metamorphic calcite marbles even under closed-system conditions. In siliceous limestones with the sedimentary assemblage Qtz + Cal and an H20-rich pore fluid, for example, wollastonite will form in small but petrographically detectable amounts at temperatures around 600°C.
The Candoglia marble
The quarries of Candoglia, Northern Italy, were firstly opened near the Candoglia village, but later their location moved up to higher levels. Now there are five recognisable extraction sites: Madre quarry (Cava Madre), active underground quarry at an elevation of 560 m a.s.l.; Carrettone quarry (Cava Carrettone), abandoned open pit quarry; Cornovo West quarry (Cava Cornovo Ovest), abandoned open pit quarry; Cornovo East quarry (Cava Cornovo Est), active open pit quarry at 860 m a.s.l.; Mergozzoni quarry (Cava Mergozzoni or Cava dei Mergozzoni), abandoned open pit quarry at 950 m a.s.l.
View of the Madre quarry (Cava Madre), Candoglia, Ivrea Verbano, Italy. From Sesia Valgrande Geopark.

Panoramic view from the Madre quarry (Cava Madre), Candoglia, Ivrea Verbano, Italy. From Duomo di Milano.
The candoglia marble consist of lenses of calcite and calc-silicate marbles, interlayered within the kinzigites of the Ivrea-Verbano Zone, with a subvertical attitude and small thickness (8-30 m). Structural studies evidenced isoclinal folds, with subvertical axial plane parallel to the main foliation, which connect the different small lenses. Since 1387 the Candoglia marble quarries belong to the "Veneranda Fabbrica del Duomo" of Milan and now the produced stone (about 1000 t/year) is only used for the restoration of the Milan cathedral. The Candoglia marble was also used in the past as facing stone in few other monuments such as Certosa in Pavia, Cappella Colleoni in Bergamo, and San Petronio Cathedral in Bologna. The medium to coarse-grained Candoglia marble mainly shows a pink colour with frequent dark green layers, due to the presence of diopside and tremolite; other subordinate minerals are quartz, epidote, sulphides, Ba-feldspars, baryte, and rare phlogopite. The varieties of Candoglia marble (pink, white, grey, and veined) are deeply affected by the chromatic, textural, and compositional features. Madre quarry (Cava Madre) is the type locality for taramellite and paracelsian; Mergozzoni quarry (Cava Mergozzoni or Cava dei Mergozzoni) is the type locality for wenkite.

Polished Candoglia marble with dark calc-silicate layers.

Polished Candoglia marble with dark calc-silicate layers.
Bibliography
• Bucher, K., & Grapes, R. (2011). Petrogenesis of metamorphic rocks. Springer Science & Business Media.
• Fossen, H. (2016). Structural geology. Cambridge University Press.
• Howie, R. A., Zussman, J., & Deer, W. (1992). An introduction to the rock-forming minerals (p. 696). Longman.
• Passchier, Cees W., Trouw, Rudolph A. J: Microtectonics (2005).
• Philpotts, A., & Ague, J. (2009). Principles of igneous and metamorphic petrology. Cambridge University Press.
• Shelley, D. (1993). Igneous and metamorphic rocks under the microscope: classification, textures, microstructures and mineral preferred-orientations.
• Vernon, R. H. & Clarke, G. L. (2008): Principles of Metamorphic Petrology. Cambridge University Press.
• Vernon, R. H. (2018). A practical guide to rock microstructure. Cambridge university press.


(1).jpg)
(2).jpg)
(3).jpg)
(4).jpg)
(5).jpg)
(6).jpg)
(7).jpg)
(8).jpg)
(9).jpg)
(10).jpg)
(11).jpg)
(12).jpg)
(14).jpg)
(15).jpg)
(16).jpg)
(17).jpg)
(18).jpg)
(19).jpg)
(22).jpg)
(23).jpg)
(24).jpg)
(25).jpg)
(26).jpg)
(27).jpg)
(28).jpg)
(29).jpg)
(30).jpg)
(31).jpg)
(32).jpg)
(33).jpg)
(36).jpg)
(37).jpg)
(38).jpg)
(39).jpg)
(40).jpg)
(48).jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)