Plagioclase - NaAlSi3O8--CaAl2Si2O8
Plagioclase feldspars are the most common feldspar minerals because calcium is somewhat more common in the crust than potassium (3.6 and 2.8 percent of the crust, respectively). Plagioclase feldspars form a continuous solid solution between Ab and An endmembers at high temperature, but the replacement of ions needs to be coupled because of the charge difference between Na+ and Ca2+. The charge balance in maintained by substituting Al3+ for Si4+. The amount of potassium that may enter the lattice is limited because of large difference in ionic radii.The plagioclase series is arbitrarily divided into six minerals or compositional ranges: albite (Ab90 - Ab100), oligoclase (Ab70 - Ab90), andesine (Ab50 - Ab70), labradorite (Ab30 - Ab50), bytownite (Ab10 - Ab30), and anorthite (Ab0 - Ab10). These boundaries have no structural significance. Their use is justified because plagioclase is very common mineral and occurs in a wide variety of rocks and the composition of plagioclase is rather predictable. For example, it is common to find sodic plagioclase (oligoclase) in granite, more calcium-rich varieties (labradorite) in mafic rocks like gabbro, and intermediate andesine in intermediate igneous rocks like andesite.
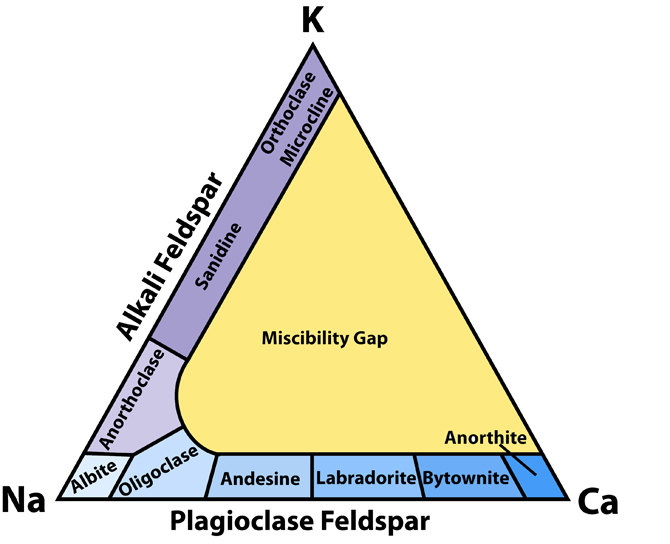
Fig.1: Feldspar classification. This diagram shows how feldspar minerals are classified on the basis of their chemical composition. The sequence of minerals along the base of the triangle represents the solid solution series of plagioclase between albite and anorthite.
Like the alkali feldspars most plagioclases are ternary solid solutions of three components: albite (Ab), anorthite (An) and orthoclase (Or). In plagioclase the orthoclase component is usually at very low concentrations. The peristerite, Bøggild and Huttenlocher intergrowths are lamellar intergrowths (Fig.2) believed to form on solvus curves. With very rare exceptions they are on sub-micrometre scales and not visible in an optica lmicroscope. Depending on their periodicity they can cause iridescence, the best known being the labradorescence caused by Bøggild intergrowths.
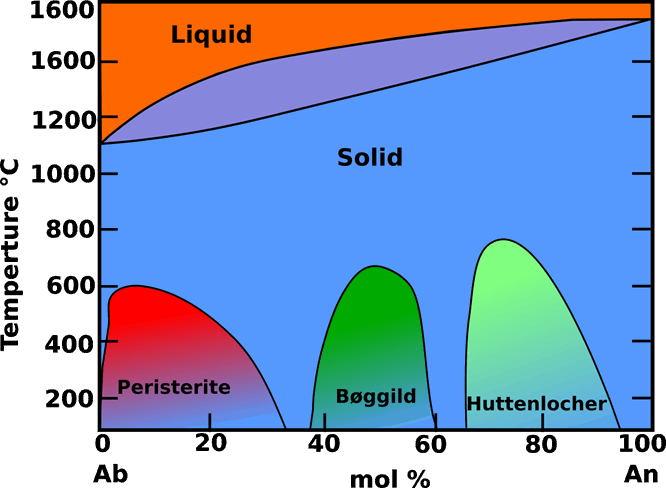
Fig. 2: Phase diagram for Or-free plagioclase feldspars at atmospheric pressure. Modified from Deer, W. A. (2001).
In thin section, plagioclase commonly shows the characteristic albite polysynthetic twinning. This twinning is the most characteristic identifying feature of plagioclase, and makes its identification easy when present. Although some cross-hatched twinning may also occur in plagioclase, it is always very simple with only one or two cross twins per grain. Thus, be careful not to identify plagioclase as microcline. The cross-hatched twinning in microcline is always much more complex.
Bibliography
• Bennett, E. N., Lissenberg, C. J., & Cashman, K. V. (2019). The significance of plagioclase textures in mid-ocean ridge basalt (Gakkel Ridge, Arctic Ocean). Contributions to Mineralogy and Petrology, 174(6), 49.
• Frost, B. R., & Frost, C. D. (2019). Essentials of igneous and metamorphic petrology. Cambridge University Press.
• Gill, R. (2011). Igneous rocks and processes: a practical guide. John Wiley & Sons.
• Winter, J. D. (2014). Principles of igneous and metamorphic petrology (p. 738). Harlow, UK: Pearson Education.


.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)