Plutonic Carbonatite
The Cape Verde Islands (Republica de Cabo Verde) comprise a horseshoe-shaped archipelago of ten main islands and numerous islets situated about 450 km west of Cape Verde, the westernmost point of West Africa. The islands are volcanic ocean islands, all lying on the Cape Verde Rise, which is supported by a thermal plume. The islands rest on Late Jurassic to Cretaceous oceanic crust. MORB pillow lavas from the oceanic crust have been uplifted by intrusive complexes on two of the islands: Maio and Sao Tiago.Of the ten islands in the archipelago, carbonatites occur on five: Brava, Fogo, Sao Tiago, Maio and Sao Vicente. The first four comprise the south-eastern group of the archipelago, and Sao Vicente is the single representative of the northern islands.
Oceanic carbonatites from the Cape Verde and Canary Islands can be divided into calcio (calcitic) – and magnesio (dolomitic) – carbonatites based on their CaO and MgO contents. The calcio-carbonatite rocks show only minor recrystallization in thin sections and have stable isotopic compositions that plot within or near the fields for primary (mantle-derived) carbonatites. In contrast to the calcio-carbonatites, the magnesiocarbonatites show extensive recrystallization accompanied by the replacement of calcite with secondary dolomite. The magnesio-carbonatites generally occur as smaller structures and are usually found in porous country rocks such as pillow lavas and breccias.
Fogo is located only 20 km east of Brava. The carbonatites of Fogo (all of which are sövites) occur in the basement, which is exposed in valleys incised in the western lower flanks of the island. Here sövites, sometimes containing abundant biotite, are cut by basic dikes and are overlain unconformably by numerous lavas coming from the main cone of Fogo. K/Ar ages of biotite from carbonatites yielded ages of 3.7 Ma.
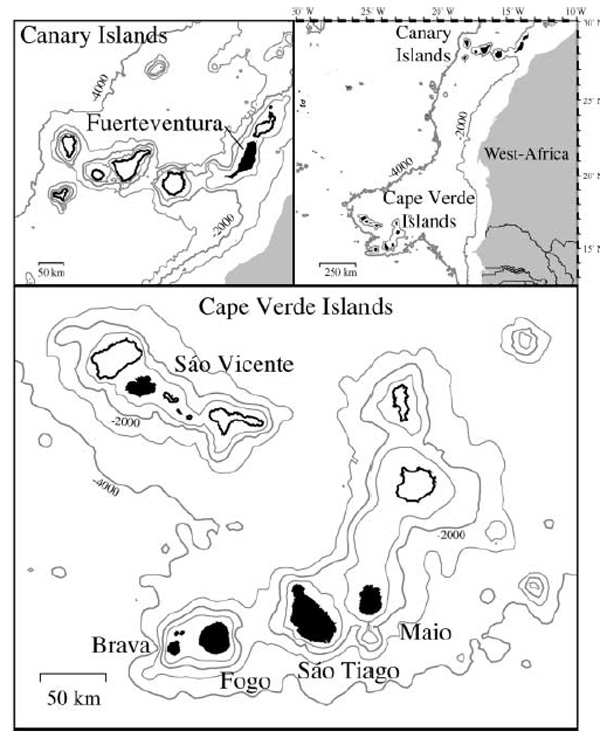
Map of the eastern North Atlantic showing the Cape Verde and Canary Islands. Islands with carbonatites are shown in black. From Kaj Hoernle (2002).
Carbonatites are commonly defined as magmatic rocks with high modal abundance of carbonate minerals (>50 wt%) and geochemistry typified by high abundances of Sr, Ba, P and the light rare-earth elements (LREE) They have been subdivided on the basis of their dominant modal carbonate mineral, such as calcite or dolomite carbonatites and on their corresponding major element geochemistry with Mg Ca, Fe- and REE-carbonatite:
Calcite-carbonatite - where the main carbonate is calcite. If the rock is coarse- grained it may be called sövite; if medium to fine-grained, alvikite
Dolomite-carbonatite - where the main carbonate is dolomite. This may also be called beforsite.
Ferrocarbonatite - where the main carbonate is iron-rich.
Natrocarbonatite - essentially composed of sodium, potassium, and calcium carbonates
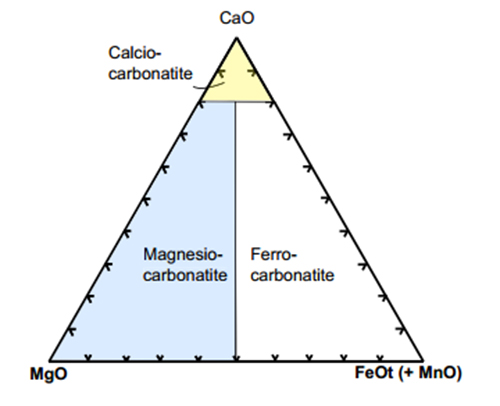
Carbonatite classification diagram; after (Woolley and Kempe 1989). Note: ferrocarbonatite can also be rich in REE.
In parallel, a process-related classification would divide them into two groups: primary carbonatites and Carbothermal residua (Mitchell 2005). In this scheme, primary carbonatites can be further divided into groups of magmatic carbonatites associated with nephelinite, melilitite, kimberlite, and specific mantle-derived silicate magmas, formed by partial melting, whereas carbothermal residua carbonatites form as low-temperature fluids rich in CO2, H2O, and fluorine.
Carbonatites characteristically occur in close association with alkalic silicate igneous rocks, either in individual complexes or in a regional association within magmatic provinces. Associated silicates are usually alkaline ultramafic rocks: pyroxenites or nephelinites (and/or ijolites) generally, but may also include more evolved types such as phonolites and nepheline syenites.
The three main theories for the origin of carbonatites are essentially:
1. Residual melts of fractionated carbonated nephelinite or melilitite
2. Immiscible melt fractions of CO2-saturated silicate melts
3. Primary mantle melts generated through partial melting of CO2-bearing peridotite
Combinations of these three theories are also popular; for example carbonatite liquids generated by deep melting of carbonated eclogite in the upper mantle infiltrate overlying peridotite to produce silica under-saturated carbonate-bearing melts, which then penetrate the crust and evolve or un-mix (Yaxley and Brey 2004).
Carbonatites have also been considered to be generated in the lithospheric mantle as partial melts rising rapidly above a hot ascending mantle plume. If these mantle carbonate melts stall, for example owing to thermal death, they generate carbonate-melt metasomatism in the mantle. As the much hotter center of the plume approaches, melting is induced in the metasomatic horizon and results in generation of the carbonatite melts that are observed on the surface. Although the plume model is quite attractive, recent recognition of strong and repeated lithospheric controls in the compilation of global carbonatite ages are thought to argue against a direct connection to mantle plumes (Woolley and Bailey 2012).
The atomic structures of carbonate melts have been little studied in comparison to the structure of silicate melts, but are fundamental in controlling their physical and chemical behavior in natural systems. Carbonate melts are ionic liquids consisting of carbonate CO32- molecular anions and metal cations that interact principally due to coulombic interactions and are thus very different from silicate melts, which have network structures characterized by polymerization (Mysen 1983). Ionic carbonate melts have been considered to be structureless with no defnite association between metal cations and carbonate molecules. However the combined evidence from phase relations of carbonates, the solubility of metals in carbonate liquids, and the spectroscopy of carbonate glasses and atomic simulations, suggests that carbonate liquids have structure at scales larger than their component molecular groups.
Bibliography
• Geochemistry of oceanic carbonatites compared with continental carbonatites: mantle recycling of oceanic crustal carbonate. Kaj Hoernle (2002), Contrib Mineral Petrol (2002)
• Intrusive carbonatites from Brava Island (Cape Verde): preliminary geochemical data. Mourao, C.
• Eric A.K.Middlemost (1985): Magmas and Magmatic Rocks. Longman, London
• Ron H. Vernon (2004): A pratical guide to rock microstructure. Cambridge editore
• K.G.Cox, J.D.Bell & R.J Pankhurst (1979): The interpretetion of igneous rocks. George Allen&Unwin editori.
• David Shelley (1983): Igneous and metamorphic rocks under the microscope. Campman & Hall editori


.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)